-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Fluorescence
Fluorescence Spectroscopy Equipment.
The NBL has an extensive suite of standard and advanced spectroscopic systems in order to sustain our wide and varied research objectives.
We have a mixture of standard steady-state spectrometers for routine analysis, several modified for polarized EEM measurements, and multiple fluorescence lifetime based systems for time-resolved studies.
We also have the capability to build our own systems as the need arises.
 |
Most of the general fluorescence equipment is located in the middle laboratory (Lab 7 in the Concourse), while the more advanced lifetime based systems are in the dedicated microscopy laboratory downstairs.
Fluorescence (steady state):
Aqualog (Horiba) |
|
This system is being configured for both high sample number experiments and flow based measurements. We've been using these to investigate the feasibility of using polarized Excitation Emission Matrix (pEEM) for the quantitative analysis of large protein complexes, vaccines, the kinetics of protein conjugation reactions, bioprocess monitoring, and the analysis of protein-polymer interactions. This has involved us fitting bespoke wire grid, UV transparent polarizers in both instruments. The pEEM approach will complement and extend the A-TEEM capability of the Aqualog, as part of our ongoing collaboration with Horiba. This combination is more correctly called: Polarised simultaneous Absorbance, Transmittance, and Fluorescence Excitation-Emission-Matrix (PolA-TEEM). The other system is on loan from Horiba and has been upgraded with a Rapid Mixing accessory (SFA-20 from TgK Scientific) for kinetic studies. This is currently being used to study the kinetics of protein-liposome interactions. |
Eclipse (Agilent). |
|
It has a front surface accessory and standard polarizers for additional functionality. In 2008 we also acquired a thermostatted multi-cell holder for high throughput studies. This system is primarily used for cell culture media studies. This system has been in continual operation for more than 14 years now and we still have not yet had to change the bulb...not bad, considering that it has collected hundred's of thousands of emission spectra. Two more Agilent Eclipse systems (2012) were fitted with wire grid polarizers polarizers for anisotropy research. We also have a multi-well accessory for these systems. A fourth system (2015) was installed for the pEEM/anisotropy based research projects. Three Ocean Insight fibre coupled spectrometers are also available and used for routine in-situ fluorescence measurements. |

We also have a Perkin-Elmer LS-50B fluorescence spectrometer which can perform a range of techniques from time resolved fluorescence, phosphorescence and chemiluminescence measurements. This is typically used for undergraduate physics and chemistry research projects. A front surface sample holder is available for fluorescence measurements of optically dense materials such as crude oils and polymers. |
 This picture above (2017) shows the middle of the PAT lab with a Shimadzu UV-1601 UV-Visible and Perkin-Elmer LS-50B spectrometers. This picture above (2017) shows the middle of the PAT lab with a Shimadzu UV-1601 UV-Visible and Perkin-Elmer LS-50B spectrometers. This workstation is generally used for the characterization of novel fluorophores and undergraduate student projects. |
Fluorescence Lifetime, cuvette systems 1 & 2:
The primary fluorescence lifetime system for routine work is a FluoTime 200 system from PicoQuant. The system was delivered in 2003 (funded by SFI) and replaces a system we had built in house (see below).
|
This system allows for the automated collection of lifetimes over the 300-850 nm range, the collection of Time-Resolved Emission Spectra (TRES) and Anisotropy data. The following excitation sources are available:
A Sepia dual channel laser driver is also available. |
 The sampling chamber (right) is large enough to accommodate a range of sampling accessories including liquid nitrogen cooled cryostats. The sampling chamber (right) is large enough to accommodate a range of sampling accessories including liquid nitrogen cooled cryostats. A Quantum Northwest TLC-50F temperature controlled sample chamber can also be retrofitted to the system for temperature dependent studies. This system was funded via a Science Foundation Ireland Principal Investigator award. |
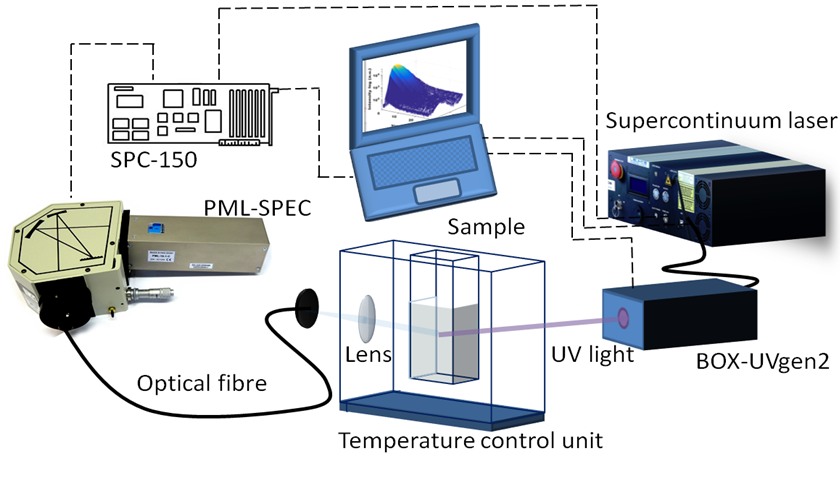
The second primary lifetime system is the Excitation-Emission Fluorescence Lifetime Spectrometer (EEFLS) which was developed and built in 2017-18.
|
This is configured for UV-visible lifetime measurements. This system was funded via a Science Foundation Ireland Principal Investigator award. |
 This system uses a supercontinuum laser source from Leukos. This system uses a supercontinuum laser source from Leukos. This is a SMHP-4W system equipped with a frequency doubler for UV generation. The system was installed in 2017 and is shown in operation here with visible light output. Tech Specs:
This system was funded via a Science Foundation Ireland Principal Investigator award. |

Fluorescence Lifetime, other systems 3, & 4:
These systems can be built up as required using either a PicoQuant TtimeHarp 100 TCSPC card or a PicoQuant PicoHarp 300 . Both of these have been recycled from earlier primary systems.
This can be coupled either to the confocal microscopes or to the various cuvette and sample holders depending on requirements.
The PicoHarp allows us to undertake Time-Tagged Time-Resolved (TTTR) measurements, as well as standard FCS, and lifetime measurements.
At present (Mar. 2025) the system is attached to a confocal microscope for FCS measurements.
Back to Top
UV-visible spectrometers
We currently have four systems in place which are mainly used for solution phase measurements.
- A Shimadzu UV-1800 double beam spectrometer was installed in 2017 to facilitate routine solution measurements of media and proteins.
- A Cary 60 single beam instrument (Installed Sept. 2015) is available for routine solution measurements using both immersion probe and cuvettes.
- We also have a dedicated high specification UV-VIS-NIR system, a Perkin Elmer Lambda 950 (Installed March 2008).
- A Shimadzu UV-1601 (~installed 2000) is used for teaching providing routine solution phase UV-Visible measurements for final year undergraduate research projects..
 The Lambda 950 system comes with a Universal Reflectance Accessory for the analysis of thin films and other solid substrates. The Lambda 950 system comes with a Universal Reflectance Accessory for the analysis of thin films and other solid substrates. We have also available a Harrick Praying Mantis diffuse reflectance accessory for the analysis of powders and solid materials. The system is configured with a range of predefined analysis routines for complex and difficult adsorption measurements. This facility was provided under the HEA funded (PRTLI-IV) National Biophotonics Imaging Platform. |
 This is the Shimadzu UV-1800 double beam spectrometer (installed in 2017) with the Osmometer on the right hand side. This is the Shimadzu UV-1800 double beam spectrometer (installed in 2017) with the Osmometer on the right hand side. Both of these systems are mostly used for media analysis. |
 This picture above (Feb. 2017) shows one of the spectroscopy stations in the middle lab with a Cary Eclipse fluorescence spectrometer and a Cary 60, single beam, UV-visible spectrometer. This picture above (Feb. 2017) shows one of the spectroscopy stations in the middle lab with a Cary Eclipse fluorescence spectrometer and a Cary 60, single beam, UV-visible spectrometer. Both are equipped with temperature control and used for solution state studies. |
Additional Extras / Sampling Systems:
Two Ocean Optics portable fluorescence spectrometers (a USB2000-FLG and a USB4000), and fibre accessories are also available for developing a range of new instrumentation/precision sampling systems for our fluorescence based research.
We have currently (Mar. 2025) coupled one of the spectrometers and the EEFLS with a TLC-50F temperature controlled cuvette holder. This will enable us to collect fluorescence steady-state and lifetime data at the same time, while running various temperature profiles.
A VGI 2000M custom built humidity chamber for microscopy and spectroscopy is also available and we use it for studying hygroscopic polymers. The system is usable over a 10-40 C temperature and 0-100% RH range and can be coupled to Raman, FT-IR, or Fluorescence spectrometers.
 |
 |
The image on the right shows the heated sample chamber with gas and water lines attached. The left hand image shows the gas /humidity controller and heating control unit. We mostly used this hooked up to an Ocean Optics spectrometer to measure spectral changes in thin polymer films as a function of humidity (see publications for details).
As we acquire more equipment and accessories I will update the webpages with details and photos.
Historical systems:
Our first ever fluorescence lifetime system was based around a PicoQuant TimeHarp 100 TCSPC module (35 ps resolution) with LED and laser diode excitation sources. The system is contained used to be housed a light proof metal box (on left of picture) and was built up over the spring and summer of 2000. The system has been dismantled and was re-engineered using fibre coupling to assemble a system suitable for the time-resolved analysis of micro scale systems or extreme environmental conditions (low temperature studies of crude oils, protein behavior on surfaces, drug eluting polymers, etc.). This new system was coupled with the TLC-50 cell and the ocean optics spectrometer to provide multi-dimensional fluorescence data.
 |
 |
The picture on the right shows the inside of the first fluorescence lifetime system we built and used until 2003. The detector (on the right) is a Single Photon Counting Avalanche Photodiode from Perkin-Elmer and has a dark count of <100 cps.
Contact
Room 213, Nanoscale Biophotonics Laboratory,
University of Galway, University Road, Galway, H91TK33, Ireland.
Tel: +353 (0)91 492943 or ext. 2943 (internal)
E-mail: alan.ryder
 @universityof galway.ie
@universityof galway.ie This page was last updated 04/03/2025








 We are currently (Mar. 2025) using two Aqualogs from
We are currently (Mar. 2025) using two Aqualogs from  This new second system also comes with a sipper accessory and is paired with a Dynamic Light Scattering (DLS) system is to support our expanding bioanalytical projects and was installed in Nov. 2024.
This new second system also comes with a sipper accessory and is paired with a Dynamic Light Scattering (DLS) system is to support our expanding bioanalytical projects and was installed in Nov. 2024.  Our other steady-state fluorescence spectrometers are the Cary Eclipses (left) the first of which was installed in July 2004.
Our other steady-state fluorescence spectrometers are the Cary Eclipses (left) the first of which was installed in July 2004. This system is based around a TimeHarp 200 TCSPC card located in a PC above the system (pictured on the right).
This system is based around a TimeHarp 200 TCSPC card located in a PC above the system (pictured on the right).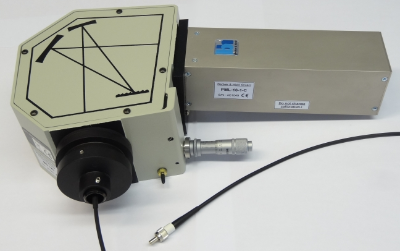 We have now also installed (2017) a Becker & Hickl
We have now also installed (2017) a Becker & Hickl 







