-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Digitally Enabled Connected Medical Devices
Digitally Enabled Connected Medical Devices
Centre for Adult Learning and Professional Development- Title of Award
- Diploma
- Course Code
- DEM1
- Delivery
- Blended Learning
- NFQ
- 8
- Award Type
- Minor
- Next Intake
- September 2026
- Duration
- 1 year, part-time
- ECTS Weighting
- 30
Why Choose This Course?
Course Information
With a balance of theory and hands-on experience, students will develop the skills needed to work with cutting-edge medical technologies in a rapidly evolving field.
Students with an industry-based project in new medical devices are encouraged to apply. Participants will be given the opportunity to develop this project concept by taking modules in Materials Science & Processes, Regulatory Compliance, Design of Engineering Systems, and Scalable Science & Technology.
At the end of the course, students will be able to:
- Identify cases where an electronic functionality can be introduced to medical devices.
- Implement a solution incorporating an electronic functionality on a medical device.
- Extract and analyse real-time data from the device for further product improvement.
- Diagnose problems by thinking critically, innovating and collaborating through team-based work.
- Evaluate, through written and oral communication, technical findings concerning connected medical devices.
- Value life-long personal and professional attributes including communication, interpersonal, analytical, enterprising and problem-solving skills in vibrant, technologically advanced economies.
The Manufacturing Process
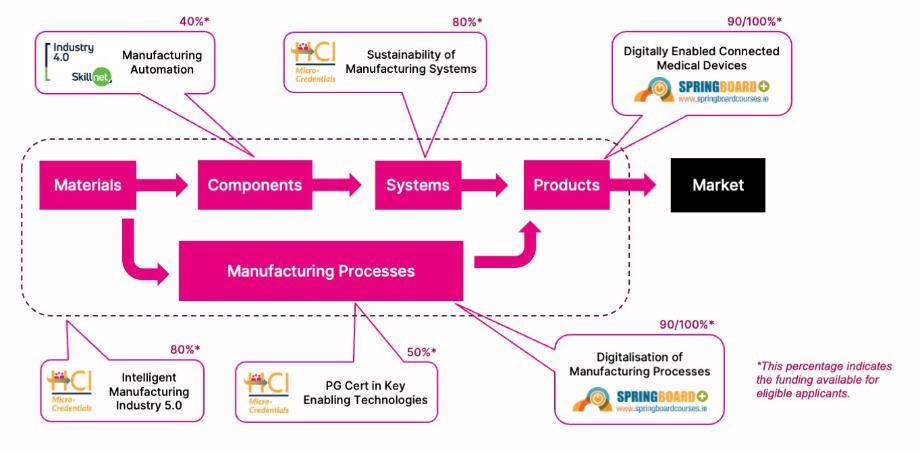
The manufacturing process involves transforming materials into functional components, which are assembled through sustainable, intelligent, manufacturing systems into products. These products are designed to meet specific market needs. Our new advanced manufacturing diplomas respond to employee skills needs at every stage of this process. See the chart below for an overview of related courses and how they align within the overall manufacturing process.
The course will consist of two 10 ECTS modules and two 5 ECTS:
10 ECTS modules:
- Materials Science & Processes with Project
This module is on the science of materials, from the small (atomic/nano level) through the micro-scale (grains, crystals, fibres etc.) to the macro or large scale (components, devices, etc.). The learner undertakes a project in materials science which includes the consideration of Atomic Bonding; Structure of Materials; Imperfections in Crystalline Solids; Diffusion; Phase Diagrams; Mechanical Properties of Materials; Failure Mechanisms & Corrosion; Processing of Materials – Polymers, Ceramics and Metals. - Design of Engineering Systems with Project
The aim of this course is to introduce the basic theoretical and practical concepts of engineering systems design. It lays out the fundamentals of a wide range of design-related activities. The module describes the major structure of design activity, highlighting the main stages of the design process, their significance, characteristics, requirements and methods of evaluation. The module describes both product and process design, design for high reliability and safety with practical emphasis on important modern problems, such as design for energy efficiency, design for low environmental impact and digitalisation. This module also aims to develop your skills in problem-solving, critical thinking, decision making, written and oral communication, teamwork and creativity, innovation and entrepreneurship.
5 ECTS modules:
- Scalable Science Technology & Innovation
This module introduces key insights on scalable science, technology and innovations. It promotes understanding of how science, technology and innovation intertwine. It helps participants to elicit the potential value of technology. A historical evolution of scientific thought and industrial development is first presented. The second part centres on understanding competition, analysing value chains, and the role of geography in innovation. The third part is technical and is focused on key technologies and patent literature. The fourth and fifth parts are strategic to the twin transition towards sustainability and digitalisation as it focuses on the formulation of a business hypothesis using approaches based on customer discovery. - Regulatory Compliance
This regulatory compliance module presents an overview of the regulations governing the development, manufacture and marketing of drugs and medical devices, inclusive of invitro diagnostic devices.
NB: You can take the modules listed above as individual standalone micro-credentials. Please see related courses section at the bottom of the webpage.

Each module features a substantial workplace project component, and students are encouraged to join the course with an industry-based project concept. This approach provides valuable opportunities to build transferable skills that can be applied directly in professional settings.
This course will suit professionals in R&D roles within the medical device sector. This course provides the commercial acumen and leadership skills needed to drive innovation and connectivity in the medical device industry by focusing on the digital transformation of manufacturing.
It is designed to equip professionals with specialised technical and soft skills for the evolving medical device sector, with a focus on connected, data-driven, and sustainable device design and manufacturing. It has been preparing learners for leadership roles in this field since 2009 and continues to evolve to meet industry demands.
The identified career pathways specific to this course include:
- Medical Device Engineer
- Healthcare Technology Specialist
- Regulatory Affairs Specialist
- Medical Device Product Manager
Dr Adam Collins
Lecturer, School of Natural Sciences, Physics
Dr Gerard O'Connor
Personal Professor, School of Natural Sciences, Physics
Dr Aidan Toner
The course is delivered part-time through a blended learning approach. Learners will be provided with online materials for each module, specifically developed for independent study. These will be complemented by recommended readings and interactive resources where relevant. In-person attendance is required for approximately 10 hours per module, typically scheduled on Saturdays.
Assessment is carried out through a combination of assignments, written examinations, and, where applicable, practical laboratory work. Examinations are scheduled at the end of each semester, and each module is assessed individually.
If you do not meet the standard entry criteria for this course, you may gain entry through our Recognition of Prior Learning (RPL) policy. Applicants may also be able to earn module exemptions based on their prior learning. Find out more here.

Job readiness is integrated across all modules through group work, problem-solving assignments, conflict resolution discussions, and leadership opportunities in team projects.
Experiential learning, including immersion in labs and opportunities for work-based projects, helps learners develop essential transversal skills such as teamwork, communication, creativity and innovation.
Accreditations & Awards
Meet our Employers
Entry Requirements and Fees
Applicants should have a Diploma at NFQ Level 7 of 90 ECTS minimum, in a Science, Engineering or Technical area, or otherwise prove that they satisfy the modules' prerequisites as listed in the course outline section.
Entry requirements for part-time students can be found here (i.e. Age, English language requirements, etc.).
This course is funded through Springboard+, you can apply for this course here.

NB: Applicants should have a Diploma at NFQ Level 7 of 90 ECTs minimum, in a Science, Engineering or Technical area, or otherwise prove that they satisfy the prerequisites of each of the modules as listed in the course outline section.
Fees for Academic Year 2026/27
| Course Type | Year | EU Tuition | Student Contribution | Non-EU Tuition | Levy | Total Fee | Total EU Fee | Total Non-EU Fee |
|---|---|---|---|---|---|---|---|---|
| UG Diploma | 1 | €2,720 | €3,220 | €- | €2,720 | €3,220 |
Course Administrator
Tel: 091 493909
Email: sciencetech@universityofgalway.ie
Why University of Galway?
World renowned research led university nestled in the vibrant heart of Galway city on Ireland's scenic West Coast.
Downloads
Meet Our Alumni
Introduction
The Diploma in Digitally Enabled Connected Medical Devices prepares students to meet industry demands by enhancing the connectivity of modern medical devices. Learners will gain essential knowledge of simple electronic systems used in medical device manufacturing while exploring smart technologies and printed electronics.
This course is funded by Springboard+.