-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Experiments
Hand warmers

You may have seen reusable hand warmers in the shops around winter time. If you’ve ever used one, you’ll know that they are small, sealed, liquid filled bags with a little metal disc inside. To make them work, all you have to do it click the metal disc and the liquid turns into a solid and gets really warm. But how do they work?
The liquid inside the bags is actually a supersaturated solution of sodium acetate. A saturated solution is one where no more material can be dissolved at room temperature. A supersaturated solution is one where the solution contains more of the dissolved material than would be possible at room temperature. For a supersaturated solution of sodium acetate to be made you heat up some water and add sodium acetate to it until no more can dissolve.
When you click the metal disc inside the bag, you shock the liquid and this shock starts what is called a crystallisation reaction. A crystallisation reaction means that crystals (like crystals of table salt) are formed. It is these crystals that turn the liquid into a solid.
This crystallisation reaction gives off heat. Reactions that give off heat are called exothermic reactions. Reactions which take in heat are called endothermic reactions. It is this exothermic reaction that helps the hand warmer to keep your hands warm on a cold day.
By reheating the pack in boiling water for a few minutes, you are dissolving the salt again. This makes the hand warmer reusable.
Why onions make you cry.
Have you ever been in tears while you're cutting up onions or even standing near someone cutting them? Ever wondered why onions make your eyes water? Let's look at the chemistry behind it.
Onions are make up of millions of tiny cells. The cells are divided into 2 sections which are separated by a membrane (a thin wall). One side of the membrane contains an enzyme and the other side of the membrane contains molecules that contain sulfur. When you cut an onion, these cells are broken and their contents are released. The enzyme and the sulfur containing molecules mix together and form volatile (goes from liquid to gas very easily) compounds called amino acid sulfoxides (sul-fox-ide). This gas gets into the air around you and reacts with the water in your eye to form sulfuric acid! Sulfuric acid is one of the components of acid rain.
The sulfuric acid irritates your eye and your eyes produce tears in order to wash it away.
Have you noticed that the watery effect stops when you cook the onion? This is because cooking the onion stops the enzyme from working so it can't produce the amino acid sulfoxides anymore.
Cutting up onions under water will also stop the tear producing reaction, as the sulfur containing compounds dissolve in water easily and will be rinsed away before they can reach your eyes. Freezing your onion for 10 minutes before you cut it will also help as cold temperatures slow down the reaction between the enzyme and the sulfur compounds.
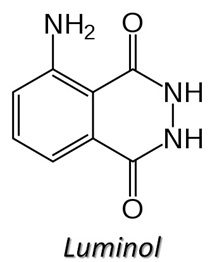
You may have seen Crime Scene Investigators on TV using a chemical called Luminol to find traces of blood at a crime scence. The investigator prepares a solution of luminol and sprays in throughout the area under investigation. The lights are then turned off and any traces of blood show up as a bright blue glow. Let's look at how Luminol works.
The luminol reacts with iron in blood to produce something called chemiluminescence (the bright blue glow). Chemiluminescence is the emission of light during a chemical reaction. The amount of iron needed to react with the luminol to produce the blue glow, is very small and this allows the detection of even trace amounts of blood.
Luminol has some disadvantages which can give the CSI guys a bit of a headache. The disadvantages are:
- Luminol chemiluminescence can be triggered by a number of substances such as copper metal and bleach. This means that if a crime scene has been cleaned with bleach, the bleach residue will cause the entire crime scene to glow blue. This camoflages any traces of blood.
- Luminol can also detect tiny traces of blood in urine and even feces and reacts the same to animal blood as to human blood.
Watch the video of luminol in the lab here at NUI, Galway.
Sacrificial Gummy Bear

The gummy bear explosion involves a reaction between potassium chlorate (KClO3) and glucose (the sugar in gummy bears). When potassium chlorate is melted, it decomposes to potassium chloride (KCl) and oxygen gas (O2).
Sugars (such as glucose, sucrose, etc.) are a great source of carbon, CO2, H2O and evergy when broken down. The energy given out is essentially our energy source when sugars are processed in our bodies.
When the gummy bear is dropped into the molten potassium chlorate, the sugars in the bear provide fuel to react with the O2 gas. This reaction is highly exothermic (gives out heat). This extra heat fuels the reaction even more, resulting in an explosion. This explosion completely annihilates the gummy bear.
Check out the video below.
Coke and Mentos geyser
I think everyone has seen a video of the Coke and Mentos Geyser, if they haven't tried it themselves (if you haven't seen a video you should go check one out!). Ever wondered how it works?
As you know, Coke is made up mostly of sugar (or sweetener), flavouring, water and preservatives. The thing that makes it fizzy is carbon dioxide gas (CO2). Until the bottle is opened and you pour some Coke out, the gas mostly stays dissolved in the water and is surrounded by water molecules. If you shake the bottle, the gas is released from the protection of the water molecules and escapes once the bottle is opened, bringing some of the Coke with it. Dropping some Mentos into the Coke also helps the CO2 gas to escape but how?
Water molecules strongly attract each other and link together very easily to form a mesh around each bubble of CO2. This attraction is called surface tension. To form a new bubble or to make a bubble bigger, the water molecules have to push away from each other. Breaking the surface tension of water requires energy. Dissolving a Mentos in the Coke provides the energy needed and means that new bubbles can form.
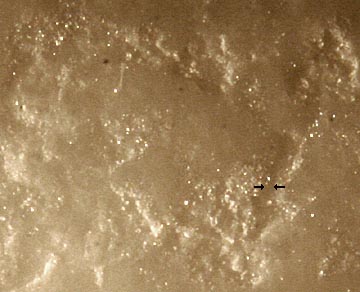
Mentos sweets have hundreds of tiny pits all over the surface (see picture above, taken from http://www.waynesthisandthat.com/mentos.htm showing the pits on the surface of a Mentos sweet) and these pits are perfect places for bubbles of CO2 to form. Add all this to the fact that the Mentos sweets are heavy and sink to the bottom of the bottle and you have a recipe for a huge eruption.
When all the gas inside the bottle is released, all of the liquid is pushed up and out of the narrow neck of the bottle creating a huge geyser.















