-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Murphy Group
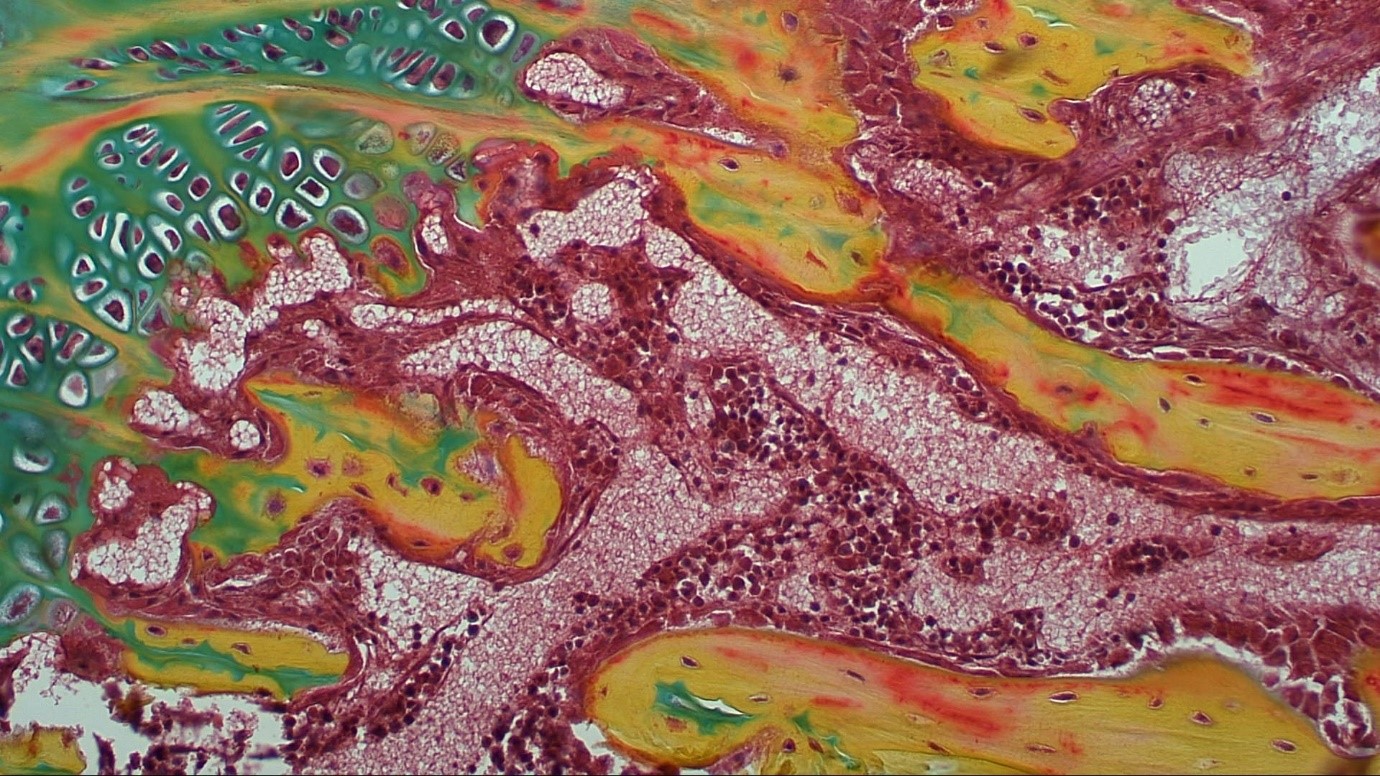
Mouse Growth plate stained with Movat Pentachrome
The Murphy group at REMEDI has a broad focus addressing:
- Basic mechanisms underpinning the use of mesenchymal stem/stromal cells (MSCs), whether derived from adult sources or induced human pluripotent stem cells (iPSCs), in disease.
- Their use as a therapeutic using both in vitro and in vivo models of disease with a specific focus on osteoarthritis (OA).
- How the cells can be used to identify marine-derived fungal extracts and purified compounds targeted at bone and cartilage repair using high throughput screening.
Translational work included:
-
Participating in the implementation of clinical trials using adipose-derived MSCs for osteoarthritis (ADIPOA1 and ADIPOA2)
- Leading projects focusing on automated production of adult MSCs (AutoSTEM) and human induced pluripotent stem cell-derived MSCs and chondrocytes) as well as characterization of the cells produced (AutoCRAT).
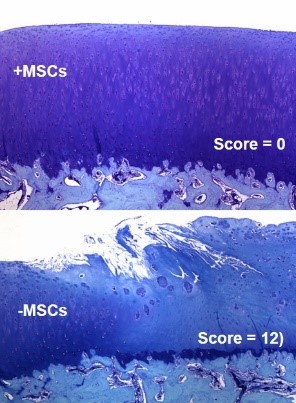
To develop novel therapies for arthritic diseases using stem cells it is critical to understand how these cells can be used to modulate disease progression or act as a therapeutic for established disease. To achieve these translational aims, it is necessary to understand the mechanisms underpinning MSCs’ capacity to treat or prevent establishment of OA and progression. Research in the group therefore focuses in the first instance on increasing understanding of the basic biology of MSCs and the mechanisms underpinning the ability of the cells to modulate disease development in vivo (Figure 1). In this context recent work has elucidated the molecular mechanisms underpinning the ability of MSCs to prevent development of OA or to treat established disease through retrieval and molecular analysis of cells that survive initial cell death after delivery to the normal or osteoarthritic mouse joint. This database represents a rich resource of identified secreted factors with the capacity to be used as a stand-alone cell-free therapy for OA and cartilage repair that will be addressed in future work by the group.
Figure 1: Allogeneic MSC treatment prevented OA progression in a pre-clinical model
The role of inflammatory mediators in establishing epigenetic changes in osteoarthritis is also under investigation. A DNA methylation array was used to identify epigenetically regulated genes associated with MSC chondrogenesis and early osteoarthritis with a targeted CRISPR-mediated strategy for validation of lead targets.
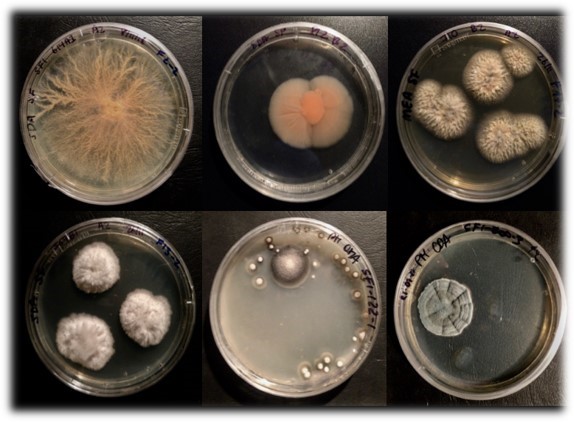
Another strategy to develop therapies for OA and bone disorders is based on the discovery of bioactive molecules able to influence MSC chondrogenesis and osteogenesis. The drug discovery program mainly focused on the detection of bioactive small molecules synthesized by marine fungi isolated from the deep North Atlantic Ocean (Figure 2). The screening program was performed using a newly designed high throughput screening platform able to detect compounds influencing MSC differentiation or acting as immunomodulators. This discovery platform can be adapted to screening of other novel extracts or purified compounds from additional sources (1).
Figure 2: Fungal species isolated from the deep North Atlantic Ocean.
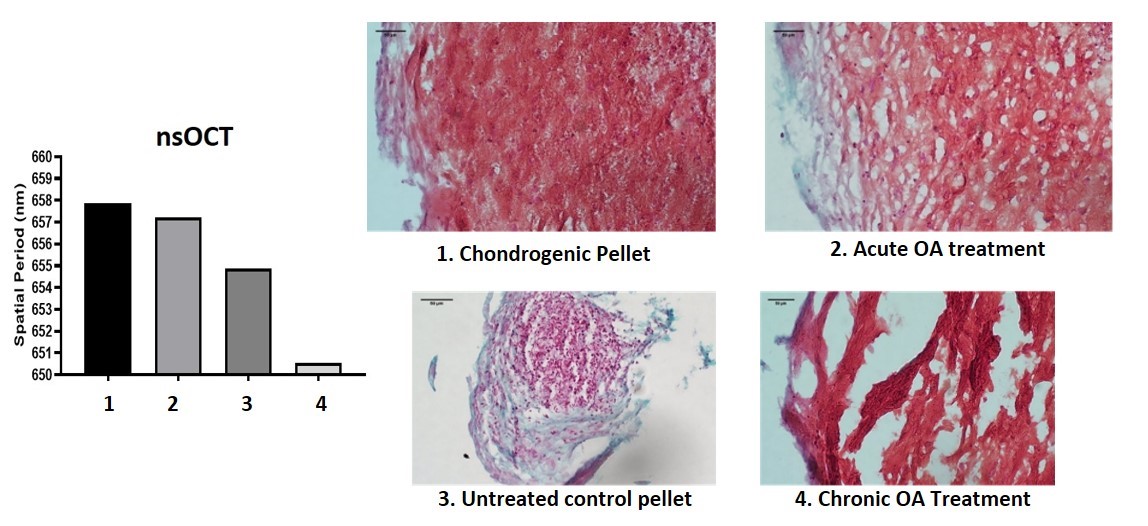
In the Starstem project an in vitro model of OA was developed to enable targeted imaging of degenerative changes associated with the disease using a novel nano-sensitive optical coherence tomography (nsOCT) developed in the Physics department at NUI Galway (https://starstem.eu) (Figure 3) (2).
Figure 3: nsOCT imaging of MSC pellets treated to induce an acute and chronic OA-like phenotype.
Applied research in the group has addressed automated production of bone marrow-derived MSCs using the AutoSTEM platform and for an online video (3).
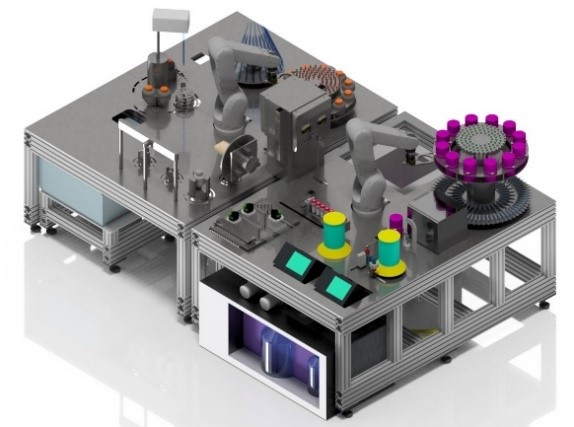
Figure 4: A representation of the AutoCRAT platform (Courtesy of the Fraunhofer Institute for Production Technology - Fraunhofer IPT, project partner)
The currently funded AutoCRAT project builds on the basic and applied research implemented in AutoSTEM to address automated production of human induced pluripotent stem cells (hiPSCs) and derived hiMSCs and chondrocytes.