-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Ryan Group
Introduction and Overview
The Ryan Tumour Immunology research group at NUI Galway focusses on understanding how the tumour microenvironment influences immune responses. We are based in the Lambe Institute for Translational Research, in an interdisciplinary environment involving immunologists, cancer researchers, Surgeons and Gastroenterologists.
Immunotherapies for the treatment of cancer, that activate endogenous anti-tumor immune responses, have revolutionised the field of oncology and shown remarkable success in some cancers. Advances in the use of immunotherapy for colorectal cancer (CRC), the second most commonly diagnosed and deadly cancer worldwide, have been largely unsuccessful. A small proportion of patients, <4% of the metastatic CRCs, with mismatch repair deficiency (dMMR) respond to immunotherapies. The other 96% of CRC patients do not respond, indicating a knowledge gap in our understanding of how these tumours regulate immune responses necessary for efficacy of immunotherapies. Understanding how the tumor microenvironment regulates immune cell infiltration and function is necessary to develop effective immunotherapeutic strategies and overcome resistance. The ultimate objective of our research is to uncover novel immune checkpoint targets to overcome iMSC/CAF immunosuppression in the TME and restore anti-tumor immunity.
We work with multiple industry collaborators, where we aim to develop novel strategies and therapy combinations to enhance immunotherapy responses in cancer patients. The key to finding new immunotherapies is in understanding how cells within the tumour communicate with suppress immune function. The Ryan Lab is funded by Science Foundation Ireland, Health Research Board, Lifetime CDT Programme, Janssen, Celgene, Bristol Myers Squibb.
Current Research Projects include:
(1) Investigation of Tumour-stromal cell interactions and subsequent effects on immune cell infiltration (Funded by SFI Starting Investigator grant)
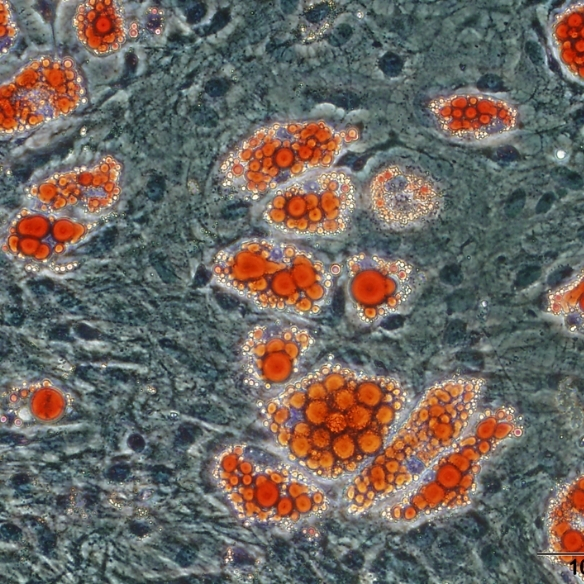
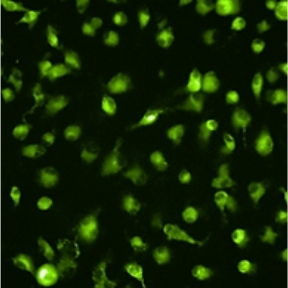
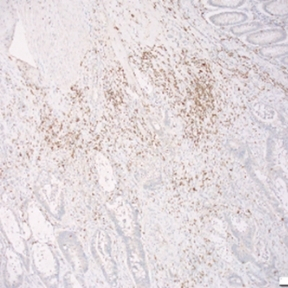
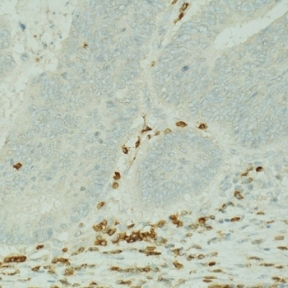
Mesenchymal stromal cells (MSCs) are multipotent stromal cells that possess immunomodulatory and pro-reparative characteristics and are currently in clinical trials as a cell therapy for the treatment of many inflammatory diseases. MSCs can acquire distinct functional phenotypes that are influenced by signals in the microenvironment, and their immunosuppressive effects have been well characterised in the context of their utility for the treatment of chronic inflammatory conditions. Data from our laboratory and others indicates that MSCs are precursors of cancer associated fibroblasts (CAFs), cells that are identified in tumours by their expression of α-SMA. Stromal cells maintain the structural integrity of tumours and impact tumour progression through multiple mechanisms, including the secretion of cytokines, and immunomodulatory mechanisms. MSC-mediated immunosuppressive effects are dependent on both cell-surface and paracrine mediators, including, programmed death ligand 1 (PD-L1), Fas ligand (FasL), prostaglandin E2 (PGE2), TGF-β and nitric oxide (NO), respectively. The focus of the research is to study the interactions between inflammation associated metastatic colon cancer cells, MSCs and the consequent influence on T cells and macrophages. We aim to identify novel immunotherapeutic targets focussed on stromal cells for the treatment of metastatic colon cancer. We have established in our laboratory a model of therapy refractory metastasis and we use imaging technologies, stromal cell expertise, and immune cell depletion strategies to further our understanding of the influence of stromal cells on anti-tumour immune responses.
(2) Sensitizing the tumour microenvironment to immunotherapeutic targeting (Funded by Janssen)
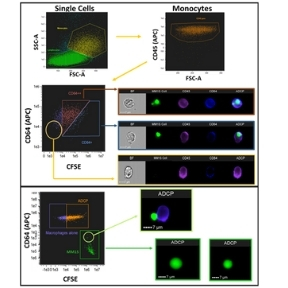
Multiple Myeloma (MM) is a malignant disorder of plasma cells which, despite significant advances in treatment, remains incurable. Daratumumab, the first CD38 directed monoclonal antibody, has shown promising activity alone and in combination with other agents for MM treatment. Daratumumab is thought to have pleiotropic mechanisms of activity including natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC). With the knowledge that CD38-expressing NK cells are depleted by daratumumab, we are currently investigating a potential mechanism of enhancing macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) by combining daratumumab with cyclophosphamide (CTX). At low doses, cyclophosphamide also has significant immunomodulatory activity, which can be used to modify the immunosuppressive tumor microenvironment in order to augment responses to existing therapies. We have discovered that cyclophosphamide induces an acute secretory activating phenotype, releasing chemokines and cytokines from treated tumour cells, which in turn induce macrophage infiltration and phagocytic activity in the bone marrow of MM patients. Current research is expanding this knowledge to other monoclonal antibody therapies in MM and CRC.
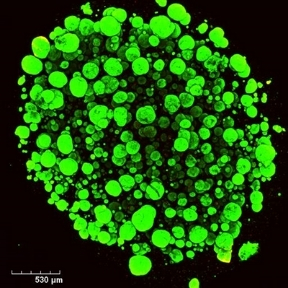
(3) Development of 3D models of the Colorectal Tumour Microenvironment (Funded by Lifetime CDT Programme)
Although there have been many advances in recent years for the treatment of colorectal cancer (CRC), it still remains the third most common cause of cancer-related deaths worldwide. Many patients with late stage CRC display resistance to multiple different therapeutics. An important aspect in developing effective therapeutics for CRC patients is understanding the interactions that take place in the tumour microenvironment (TME), as it has been shown to contribute to immune suppression and drug resistance in vivo. Using approaches such as the GelMA 3D platform, as well as dynamic microfluidic devices, we aim determine the influence of stromal cell interactions on CRC spheroid growth, as well as effects on infiltrating immune cells. We access patient samples to identify immunosuppressive features in the tumour microenvironment; and then use these features to inform the development of 3D multi-cell models to investigate novel ways to improve immune cell activation. We aim to assess how different 3D models may be optimised to study cellular and extracellular interactions that take place in the TME of CRC in an effort to allow the development of more translatable effective treatment options for patients.
Please see here for research publications.
Funding
- Irish Cancer Society
- Science Foundation Ireland (SFI)
- Galway University Foundation (GUF)
- Bristol-Myers Squibb
- Janssen
- Health Research Board (HRB)