-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Schlosser Group
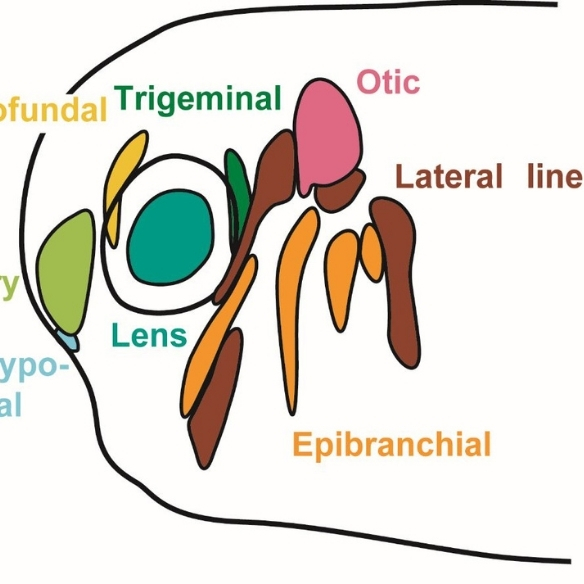
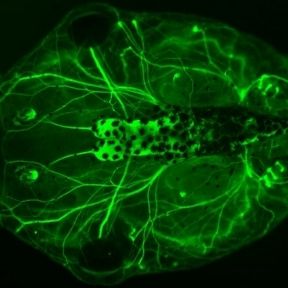
We are interested in the development and evolution of the vertebrate nervous system and sense organs. One major focus of our research are cranial placodes, the precursors of most ganglia and sense organs in the vertebrate head. We mainly use the clawed toad Xenopus laevis as a model organism to study their development. In addition, we try to elucidate evolutionary origins and modifications of cranial sense organs by comparison with other chordates. We also pursue broader questions of sensory development and evolution as well as more general theoretical problems concerning the evolution of developmental processes.
Cell fate decisions underlying specification of neural crest and cranial placodes at the neural plate border in vertebrate embryos
The evolutionary success of vertebrates is largely due to their complex head with a large brain, protected by a cartilaginous and bony skull and many intricate sense organs. Most of the specialized sensory and skeletal structures of the vertebrate head only evolved in the vertebrate lineage and they develop from two specialized embryonic tissues, the neural crest (NC) and the cranial placodes (CP) [1, 2]. Although these tissues ultimately give rise to different cell types and structures (with the NC contributing to the skull, pigment cells, glia cells and sensory neurons, and CP forming sense organs and another population of sensory neurons), they arise in the embryo from immediately adjacent regions of the neural border (the region of ectoderm located between the prospective central nervous system and the epidermis). In humans, many birth defects and other diseases are associated with cranial skeletal deformations and/or sensory deficits. While some underlying genetic causes are known, we know very little about the earliest steps of NC and CP development. This lack of knowledge seriously hampers much needed progress in developing new therapies of cranial skeletal and sensory diseases. The objective of this project is to gain new insight into how cell fate decision at the neural plate border are regulated in early embryos and which transcription factors (TF) promote one over the other fate [3]. For this purpose we will study the effect of gain and loss of candidate TFs on the expression of NC versus CP specific genes using high throughput mRNA sequencing (RNA-Seq).
Role of Six1 and Eya1 in vertebrate cranial sensory and neuronal development
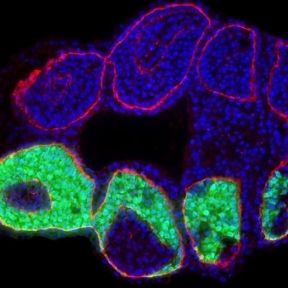
The genes encoding transcription factor Six1 and its cofactor Six1 and Eya1 play central roles in the development of sensory cells and neurons from cranial placodes. In previous studies, we cloned the Xenopus Eya1 gene and did the first comprehensive functional analyses of upstream regulators of Eya1 and Six1 in Xenopus placodes [3]. In recent studies, we used RNA-Seq of the placodal transcriptome after injection of hormone-inducible constructs of Eya1 and Six1 to identify putative Eya1 and Six1 target genes [4]. We also analysed the role of Eya1 and Six1 in regulating neurogenesis in placodes and were able to show that they can promote either progenitor states or neuronal differentiation in a context-dependent way. Interactions with different cofactors most likely contribute to these context-dependent effects Schlosser [5, 6]. After identifying multiple candidate protein interaction partners of Eya1 in a recent yeast two-hybrid screen, we currently study the function of these putative Eya1 protein interaction partners for the development of sensory cells and neurons from cranial placodes. In humans, a wide spectrum of diseases is associated with sensory deficits but our lack of knowledge regarding how sensory neurons develop hampers progress in developing therapies. In investigating molecular pathways that regulate the development of sensory neurons in vertebrate embryos, this project will helping us to understand the causes of these diseases.
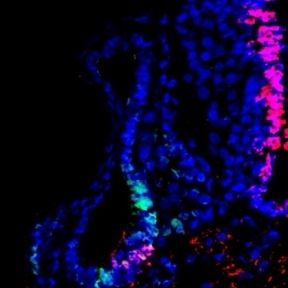
Spinal cord regeneration in Xenopus laevis
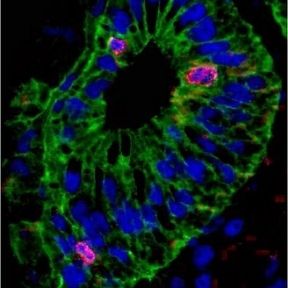
Like other mammals, humans cannot regenerate the spinal cord after injury. However. many other vertebrates are able to regenerate their spinal cord after injury. The African clawed toad Xenopus laevis provides an excellent model for elucidating the underlying mechanisms, since it offers the possibility to compare larvae which are able to regenerate the spinal cord after injury with froglets which have lost this ability. In collaboration with Abhay Pandit's lab in Cúram, my lab recently conducted a proteomic screen to identify multiple proteins that are differentially up- or downregulated in regenerative vs. non-regenerative stages of Xenopus after spinal cord injury. Ina current project, we characterize the function of some of these proteins further in gain and loss of function experiments.
Please see here for research publications.
Funding
- Science Foundation Ireland (SFI)
- EU: Marie-Curie ITN
- Irish Research Council (IRC)